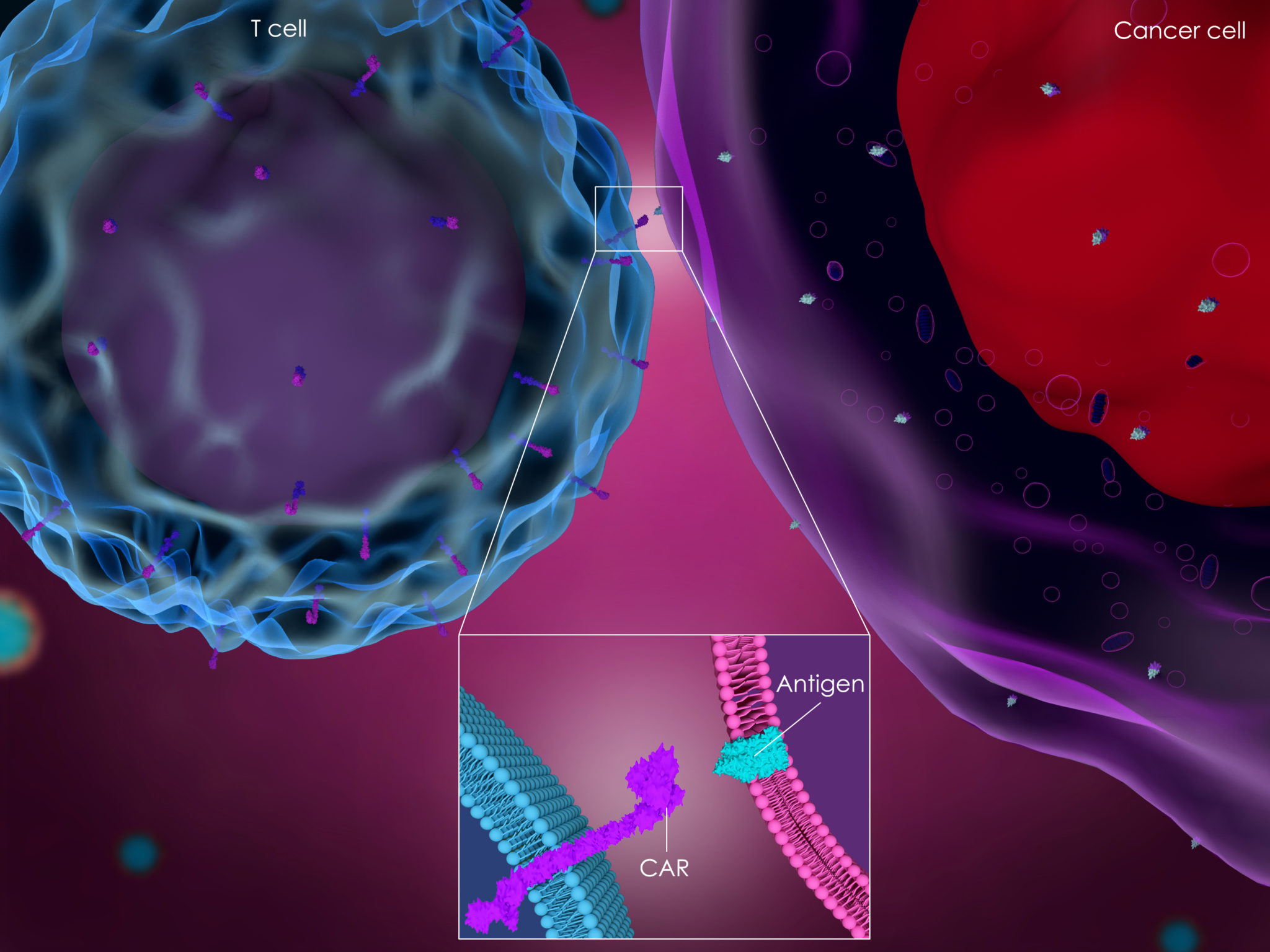
Treatment decisions in mantle cell lymphoma (MCL) have become more nuanced since the FDA approval of the CAR T-cell therapy brexucabtagene autoleucel (Tecartus) in the relapsed/refractory setting, explained Alan P. Z. Skarbnik, MD.
Previously, the treatment of choice for patients who recurred after high-dose therapy and transplant had been 1 of the 3 FDA-approved BTK inhibitors: ibrutinib (Imbruvica), acalabrutinib (Calquence), and zanubrutinib (Brukinsa). Now, there is a decision-making process between BTK inhibitors and CAR T-cell therapy, which is driven by the time to relapse, disease burden, and overall performance status of the patient.
“If the patient recurs 8 or 9 years after transplantation and does not have a lot of disease, the BTK inhibitor may be a better choice at that point. This way, you can protect the risks of CAR T-cell therapy at a later time. I have a number of patients in this setting who have very long remissions or disease control with BTK inhibitors,” said Skarbnik. “Patients who recur shortly after transplantation who have a larger proportion of the disease and are more symptomatic may be better candidates for CAR T-cell therapy.”
In an interview with OncLive®, Skarbnik, a hematologist/oncologist with Novant Health, discussed the data with available treatment options for relapsed/refractory MCL and shared some of the treatment options.
OncLive®: How do you approach the treatment selection of 3 FDA-approved BTK inhibitors in MCL?
Skarbnik: We did not have a head-to-head comparison trial between the three agents. All three agents are available and efficient. All we have is cross-trial comparison. The duration of the [DOR] response in the acalabrutinib trial appears to be longer. However, patients were in earlier treatment lines; the median number of previous treatment lines was 2 vs. 3 in the ibrutinib trial. This may contribute to the DOR difference. Zanubrutinib has a very good DOR and a deep response.
All three drugs seem to be safe and well tolerated. They have different safety profiles, which is not to say] that one is better than the other but [rather] that you have to determine what adverse effects [AEs] are best tolerated in a particular patient.
Head-to-head comparisons are made between zanubrutinib and ibrutinib in Waldenström macroglobulinemia. As far as the safety profile is concerned, zanubrutinib appears to be better tolerated in that particular trial and in that particular disease. You can extrapolate this to other conditions of the disease, because the mechanism of action of the drugs is the same throughout. Newer second-generation BTK inhibitors may be better tolerated than the first-generation BTK inhibitor ibrutinib, but we can’t say that with certainty. All three drugs are great. [The decision will come down to the choice of a physician.
How has the paradigm been affected by CAR T-cell therapy?
Brexucabtagene autoleucel was recently approved in MCL based on data from the ZUMA-2 study, which evaluated this treatment in patients with recurrent, relapsed/refractory MCL who had previously been exposed to a BTK inhibitor. However, approval did not require patients to be exposed to a BTK inhibitor in a commercial setting. It is now approved for second-line or [later] use which is appropriate at this point, because although BTK inhibitors are a great treatment option for patients with relapsed MCL, the best DOR we have seen so far is close to 26 months, which is certainly not ideal.
We try to treat MCL more aggressively in the frontline setting with high-dose cytarabine-containing induction regimens followed by high-dose chemotherapy and autologous stem cell transplantation or rescue. This appears to give patients a longer remission and progression-free survival than other frontline treatment approaches.
The main concern here is for patients who recur shortly after this intensive treatment—those with recurrent disease 1 to 3 years after a stem cell transplant. These patients are at higher risk of resistance to second-line therapy and are at higher risk of early progression following second-line therapy. Until now the treatment of choice has been BTK inhibitors, which are easy to take and readily available. The AEs are well tolerated and [patients] have a response [to this type of treatment].
The length of the response here is the issue. We now have the option of CAR T-cell therapy in this setting. Again, this will depend on how soon the patient relapses after the transplant and their disease burden. We also need to consider the overall performance status of the patient. Will they be a good candidate to withstand the AEs of CAR T-cell therapy, or will they be a better candidate for a BTK inhibitor?
We have not yet had long-term follow-up to the [ZUMA-2] trial. The true long-term efficacy and disease effect of CAR T-cell therapy is not yet known. We don’t know if using CAR T-cell therapy before a BTK inhibitor changes the response rate or DOR. We don’t have that kind of data. It’s something that we’re going to have to look back at retrospectively and try to compare as best we can.
Certainly, I would be more likely to use CAR T-cell therapy in a younger patient who has early recurrence after frontline induction, especially if they are transplanted, and certainly for those who recur after BTK inhibitors. Very few agents are able to save a patient [after that]. In terms of chemotherapy, [rituximab/bendamustine with low-dose cytarabine] appears to be a chemotherapy regimen with better response rates in the BTK inhibitor-refractory or relapsed setting. However, CAR T-cell therapy has been specifically studied in this setting, so it is a good option for those patients.
Ref: onclive.com